Mitochondrial dysfunction in ovarian cancer
Dorota Gumiela1,2
 Affiliacja i adres do korespondencji
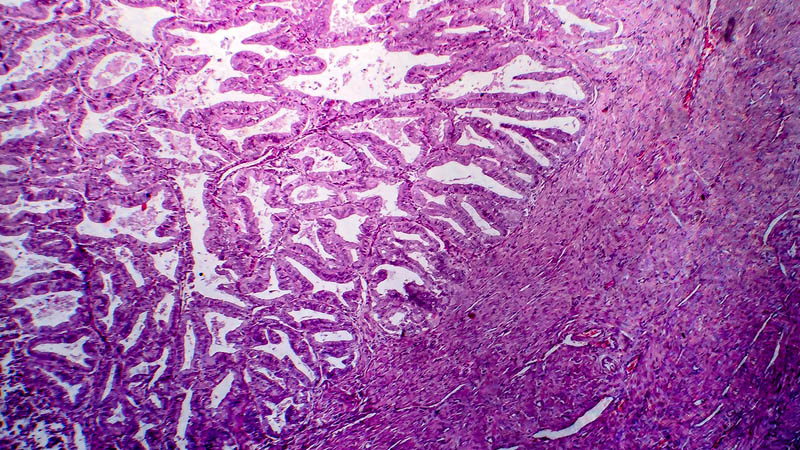
Affiliacja i adres do korespondencjiMitochondria are present in almost all eukaryotic cells, except for red blood cells, and are primarily responsible for the production of ATP, which is a product of aerobic respiration. Mitochondrial DNA (mtDNA) is a circular molecule composed of 16,596 base pairs and responsible for coding 37 of all 25,000 genes. Mutations impair energy efficiency of mitochondria, and ultimately the functioning of cells and tissues. In mtDNA, mutations take place faster than in nuclear DNA. The reason for the increased rate of mutation in mtDNA is higher exposure to reactive oxygen species that arise from oxidative phosphorylation and damage unprotected mtDNA histone proteins. The first discovery suggesting a link of cancerous diseases with mitochondrial damage was the observation of the shift of the respiration process towards glycolysis. Cancer cells actively metabolize glucose to lactic acid, without the use of oxygen despite its presence. Respiratory chain defects may be associated with the formation of free radicals and an increase in oxidative stress in cancer cells. Mutations in mitochondrial DNA are most often T→C or G→A transitions and are limited to four regions of the mitochondrial genome: the D-loop, 12S rRNA, 16S rRNA and cytochrome b. Mitochondrial DNA dysfunctions are observed in women with ovarian cancer and in other disease entities, and the use of chemotherapy seems to damage mtDNA to a small degree. The modification of the currently used chemotherapy in patients with ovarian cancer can contribute to an increase in mtDNA damage, resulting in improved treatment efficacy and longer survival.