Treatment with bevacizumab in ovarian cancer – clinical trials review
Jolanta Lubin1, Anna Markowska2, Janina Markowska1
 Affiliacja i adres do korespondencji
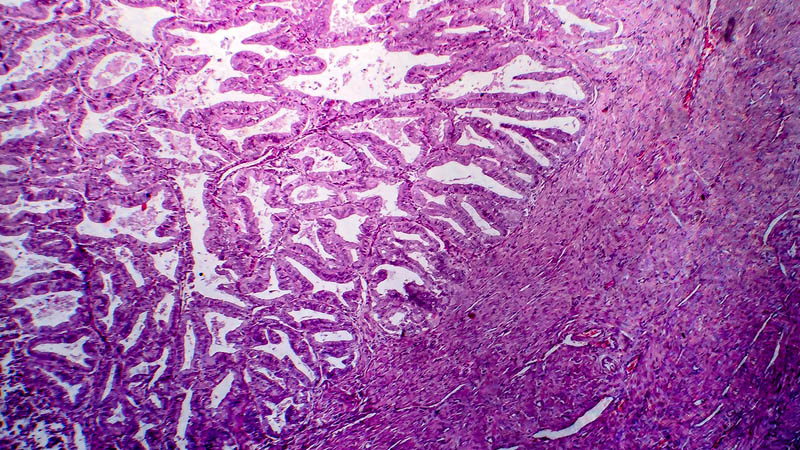
Affiliacja i adres do korespondencjiAngiogenesis is a process which has been in the focus in the last decade due to its role in the development of cancers. The identification of vascular endothelial growth factor (VEGF) in the beginning of the 80’s became the new objective of therapies of cancers. The finding of angiogenesis inhibitors has brought the therapy of cancers including ovarian cancer in a new era. Apart from the “golden standard” such as the taxans and platinum components, the treatment of ovarian cancer has not introduced any new front-line drugs for many years. The registration of bevacizumab has changed data regarding in particular the progression-free survival (PFS), which was confirmed by GOG-0218 and ICON-7 clinical trials. The aim of the article was to put clinical trials that pointed to the efficiency of bevacizumab in order as well as presenting those whose results may allow in the nearest future for a wider use of bevacizumab – not only in the front-line chemotherapy of ovarian cancer, but also in neoadjuvant therapy and the treatment of a recurrent neoplasm, both platinum-sensitive and platinum-resistant. Studies of predictive factors, which allow for a group of patients who benefit most from anti-angiogenic drugs treatment to be singled out, are also important. At present, clinical trials and scientific studies are under way and maybe they will help identify patients whose PFS and the overall survival (OS) time is be prolonged as a result of treatment with bevacizumab.