Adjuvant chemotherapy of ovarian cancer using paclitaxel/cisplatin protocol in women over 70 – analysis of treatment course and toxicity
Paweł Stanirowski1, Aleksandra Zyguła1, Agnieszka Nalewczyńska2, Krzysztof Cendrowski2
 Affiliacja i adres do korespondencji
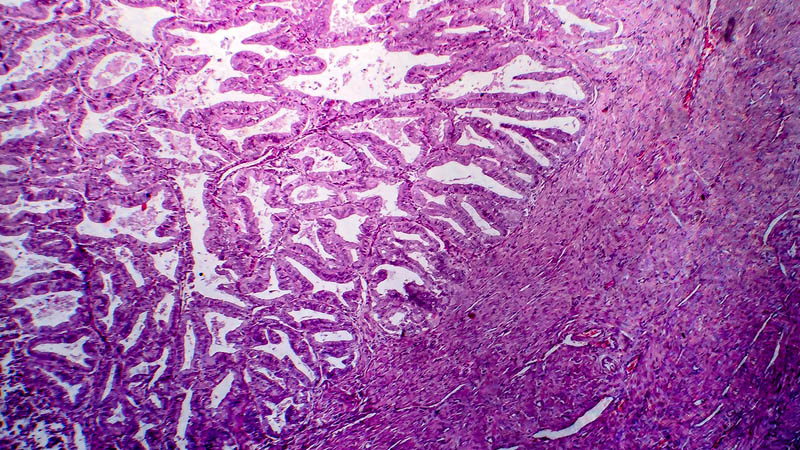
Affiliacja i adres do korespondencjiAim of paper: Analysis of clinical course of 1 st line chemotherapy acc. to paclitaxel/cisplatin protocol in women over 70 with ovarian cancer. Secondary endpoint was treatment-related toxicity in this population of patients. Material and method: This retrospective study included 25 patients over 70 with confirmed diagnosis of ovarian cancer at the beginning of their therapy (study group) and 25 patients under 70 continuing their therapy (control group). All patients underwent primary surgery and received adjuvant chemotherapy (paclitaxel 135 mg/m2 and cisplatin 75 mg/m2). Treatment-related toxicity was assessed using Common Terminology Criteria for Adverse Events v. 3.0 (CTCAE). Results: Patients in both groups did not differ significantly in clinical stage of their disease. Women in the study group presented a significantly higher proportion of poorly differentiated ovarian cancer as compared with controls. Baseline mean CA-125 level was similar in both groups. No significant intergroup differences occurred concerning the number of chemotherapy cycles delivered or mortality rate during the therapy. Proportion of patients requiring delay or cessation of treatment was similar in both groups. No significant difference was observed in remission rates between study and control groups. Incidence of hematologic complications, renal failure, neurotoxicity and hepatotoxicity was similar in both groups. No cardiologic complications were noticed in this population of patients and proportion of patients who required blood transfusion was similar in both groups. Conclusions: Course of treatment, toxicity and clinical response are not age-dependent in patients with ovarian cancer receiving paclitaxel/cisplatin protocol.